Research strategy
Three complementary approaches / three scales of involvement of the Biotherapy-Bioproduction Integrator in research.
Development & innovation
MTInov, via the UTCT (CHRU of Nancy) and the LRGP (CNRS, University of Lorraine) is involved in a number of projects funded by public research: INTERREG, LUE, ANR, PRTK, Grand défi “Biomédicaments”, AMI “Nouvelles biothérapies et outils de production”…
These collaborations allow MTInov to ensure continuity between research and pre-clinical development, technology transfer and scale-up, as well as transfer to clinical grade in order to optimise production performance, while building a solid national and international network in biomanufacturing (in particular bioprocesses) and biotherapies.
Find out more about recent projects.
Find out more about ongoing projects.

Some key projects:
- Development of mesenchymal stem cell cultures in bioreactors (Interreg Improve-Stem project) and on-line and real-time cell monitoring (InExpanCell).
- Development of anti-SAR-Cov-2 T cells (CTL-COVID19) for patients with Acute Respiratory Distress Syndrome with persistent lymphopenia.
- ExpiNKT (PRTK): production of iNKT cells at therapeutic scale.
- Project to develop CAR T cells from a defined population such as anti-viral CTLs (CAR-CTL) in partnership with the University of North Carolina (USA).
- Design of bioreactors for human MSC culture (ANR Stemcreator).

Clinical research
The UTCT (CHRU of Nancy) is actively involved in clinical research in France and in Europe with phase II and phase III clinical trials (ANSM).
These contributions ensure that MTInov is able to master the constraints related to Good Manufacturing Practices (GMP), and to take into account early in the development phase of a process the potential barriers related to the transfer into clinical grade.

Some key projects:
- Production of umbilical cord mesenchymal stem cells (MSC) for the indication of septic shock (CHOC-MSC), for the indication of Acute Respiratory Distress Syndrome secondary to COVID19 (MSC-COVID) and Production of autologous bone marrow MSC for erectile dysfunction (MESERIC).
- Production of anti-viral T cells for the treatment of post-transplant infections in the European TRACE trial.
Industrial collaborations
The LGRP (CNRS, University of Lorraine) provides its expertise (intensification of cultivation processes, numerical simulations to characterise cultivation conditions, etc.) in the course of services and partnerships with the industrial sector, involving start-ups, small and medium-sized enterprises and large groups.
These experiences allow MTInov to be as close as possible to the needs and constraints related to the industrialisation of processes and products.

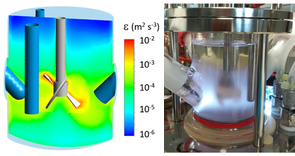
Some key projects:
- Numerical simulation of flows in bioreactors (Baxter Biosciences, Sanofi-Pasteur, Sanofi).
- Optimisation of MSC cell expansion (Vetbiobank, GPC, Bio-Inox).
- Improvement of cell culture media (Biospringer-Lesaffre, Procelys).
- Production line control (Sanofi-Pasteur, Indatech, Ondalys, Hamilton).
